Olaf A. Hougen Symposium, 2003
ADVANCES IN FUEL CELL SCIENCE AND TECHNOLOGY
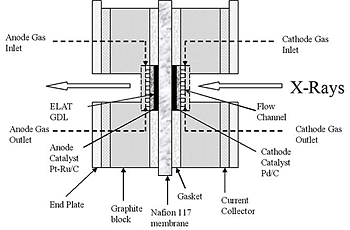
Schematic diagram of the first fuel cell studied at the Argonne National Laboratory Advanced Photon Source, courtesy of Eugene Smotkin.
Fuel cells are receiving widespread attention in academia, industry and government. The technology is among the most promising for efficient, pollution-free and noiseless energy production. Fuel cells can operate under a variety of conditions and use several alternative fuels, including hydrogen and methanol, and the electrochemical nature of fuel cell power generation allows them to circumvent the thermodynamic limitations of the traditional internal combustion engine. There are many challenges, however, in bringing fuel cells into large-scale use. This first Olaf A. Hougen Symposium brings together leading researchers to share with the UW-Madison community their research and perspectives on the science and technology of fuel cells, from the atomic to the systems engineering scale.
Schedule of Events
| Wednesday, September 17, 2003 | |
| 6:00 p.m. | Reception and dinner; Peter Hoffmann, speaker |
| Thursday, Septmeber 18, 2003, Public lectures in 1610 Engineering Hall | |
| 9:00 a.m. | Andrzej Wieckowski Mechanisms and Structure Involved in Methanol Oxidation on Pt/Ru Electrodes |
| 9:50 a.m. | Philip N. Ross Electronic and Microstructure Effects on the Electrocatalytic Properties of Platinum and Palladium bimetallic Systems |
| 10:40 a.m. | Break |
| 11:00 a.m. | Thomas A. Zawodzinski Proton Transport in Fuel Cell Membranes: Past, Present and Future |
| 11:50 a.m. | Break for lunch |
| 1:10 p.m. | Eugene S. Smotkin The development of combinatorial methods for fuel cell electrocatalysis |
| Catalysis at UW for Fuel Cell Applications |
|
| 2:00 p.m. | Manos Mavrikakis Can Electronic Structure Calculations Aid the Rational Design of Catalysts? |
| 2:30 p.m. | James Dumesic Hydrogen from Catalytic Reforming of Biomass-derived Hydrocarbons in Liquid Water |
Speakers
 |
PETER HOFFMANN is the editor of The Hydrogen & Fuel Cell Letter, which covers events and developments in this emerging field. He is a former Washington correspondent for McGraw-Hill World News. His articles on energy from hydrogen have appeared in publications worldwide, including Business Week and the Financial Times European Energy Report. Hoffmann's latest book, Tomorrow's Energy: Hydrogen–Fuel Cells and the Prospects for a Cleaner Planet (MIT Press) appeared in bookstores recently. It is an extensive update and revision of his 1981 book, The Forever Fuel — The Story of Hydrogen (Westview Press), which was called "the book on the subject" by Kirkus Review
 |
ANDRZEJ WIECKOWSKI is Professor of Chemistry at the University of Illinois at Urbana-Champaign and North American Editor for Electrochimica Acta. Professor Wieckowski has received several national (Polish and American), and international chemistry awards, including the 2003 David C. Grahame Award of the Physical Electrochemistry Division of the Electrochemical Society. His research interests are in electrochemical surface science (atomic and molecular adsorption), electrocatalysis, corrosion ultra-high vacuum spectroscopies, and in the application of metal NMR and radioactive labeling for surface studies in electrochemistry.
 |
PHILIP N. ROSS is Principal Investigator and Senior Scientist at the Lawrence Berkeley National Laboratory (LBNL). Prior to joining LBNL in 1978, he was at the United Technologies Corporation where he was a group leader in the fuel cell development programs from 1972 to 1978. Dr. Ross was one of the pioneers in the application of UHV surface analytical tools to the study of electrochemical systems, and the use of a surface science approach to the development of electrocatalysts. More recently he has advocated a surface science approach to the study of lithium surface chemistry and the lithium/electrolyte interface to advance lithium battery technology.
 |
THOMAS A. ZAWODZINSKI recently joined Case Western Reserve University as Professor of Chemical Engineering and F. Alex Nason Chair in Engineering. He is the Ohio Eminent Scholar in Fuel Cells and Director of the Case Advanced Power Institute. He also leads DOE's research activity in high temperature membranes for fuel cells. Before joining CWRU, Dr. Zawodzinski directed a comprehensive fuel cell program at Los Alamos National Laboratories with research and develeopment components in automotive, stationary and portable power applications of PEM and direct methanol fuel cells. Earlier, he led the LANL team optimizing reformate/air fuel cells, for which he received the DOE Fuel Cell Award in 1999.
 |
EUGENE S. SMOTKIN is Associate Professor of Chemistry at the University of Puerto Rico at Rio Piedras and Research Professor of Chemical Engineering at the Illinois Institute of Technology. He pioneered the in-situ synchrotron study of high performance reformate fuel cells and in-situ FTIR of DMFCs and reformate fuel cells. In 1999, he founded NuVant Systems Inc. to facilitate the combinatorial discovery of fuel cell catalysts and develop fuel cell electrolytes for operation in the "gap" region (250°C – 400°C). Dr. Smotkin's research efforts range from density functional theory calculations of catalytic systems, to the discovery of new electrocatalysts, to optimization of flow fields for single cell and array fuel cell systems.
 |
Professor
Department of Chemical & Biological Engineering
University of Wisconsin - Madison
 |
Professor
Department of Chemical & Biological Engineering
University of Wisconsin-Madison
Symposium Abstracts
Andrzej Wieckowski
Mechanisms and Structures in Methanol Oxidation on the Pt and Pt/Ru Electrodes
Methanol is an archetypical fuel molecule used in organic fuel cells. On Pt and Pt-based catalysts, which are used for fabrication of fuel cell anodes, methanol decomposes to CO, which is then oxidized to CO2. Evidence has been presented that the first C-H bond break is the rate-determining step in the CO formation path. However, under more realistic fuel cell conditions, CO removal from the catalyst surface is overwhelmingly rate determining. In addition to formation of CO2 as the end product of the oxidation reaction, there is another path leading to formation of less oxidized species: formic acid and/or formaldehyde. The path for CO2 formation without the chemisorbed CO intermediate is also considered. Therefore, the overall methanol oxidation mechanism is complex, may go via a dual/multiple path, is strongly surface-structure sensitive, and needs to be approached analytically and on the molecular level.
Examples of our most representative research on the methanol oxidation mechanism, both on pure Pt and Pt/Ru surfaces will be presented. We will first describe our method of fast voltammetric pulses and chronoamperometric measurements to elucidate the dual path mechanism on the Pt(111) electrode in the real methanol decomposition/oxidation time. We will next summarize our results on structure and phenomena involved in CO formation and CO oxidation processes. Selected model surfaces for well-defined catalysts are Pt(111) and Pt(111)/Ru, and the CO surface science on both Pt(111) and Pt(111)/Ru electrodes will be demonstrated. A nonelectrochemical method chosen to investigate molecular-level properties of CO on the Pt(111)/Ru electrode is Sum Frequency Generation (SFG). SFG spectra as a function of electrode potential and ruthenium coverage will be presented, highlighting further perspectives for the use of this technique for basic research on organic molecule fuel cells. We will then move to the illustration of the methanol decomposition mechanism on nanoparticle electrodes. While this part of my talk will mostly be dedicated to results obtained by electrochemical NMR (EC-NMR), the significance of STM images on platinum single crystal surfaces, as affecting interpretations on the nanoparticle surface, will be demonstrated. We will offer a coherent picture of methanol reactivity and chemisorption product formation on single crystal and nanoparticle surfaces for use in modeling and catalyst syntheses for the direct oxidation methanol fuel cell (DMFC).
Philip N. Ross
Electronic and Microstructure Effects on the Electrocatalytic Properties of Pt and Pd Bimetallic Systems
Progress in understanding how the electrocatalytic properties of Pt and Pd electrodes are affected by surface microstructure and electronic modification will be reviewed. The emphasis is on the study of well-characterized model electrocatalysts prepared in UHV and characterized with ex-situ surface spectroscopies. The electrode reactions discussed include hydrogen oxidation/evolution, oxygen reduction, and the electrooxidation of C1 compounds (carbon monoxide, formic acid, and methanol). The discussion will focus on the relation between the energetics of adsorption of intermediates and the reaction pathway and kinetics, and how the energetics and kinetics are effected by extrinsic properties of the model electrodes, e.g., surface structure and electronic properties. It is shown that Pt single crystals and well-characterized Pt bimetallic bulk alloys have been used with some success as models for real (i.e., commercial) catalysts. Finally, some limitations of these model systems are discussed and some new directions for studying more realistic systems with the same rigor are suggested.
Eugene S. Smotkin
The Development of Combinatorial Methods for Fuel Cell Catalysis
The application of combinatorial methods to fuel cell electrocatalysis was inspired by advances in the pharmaceutical industry, where very large libraries of discreet molecules were prepared, for example, by split and pool methods with tagging to enable post screen identification of positive hits. The subtle differences between the characteristics of discreet molecule libraries and those of mixed phase materials for catalysis, tremendously diminishes the analogies between combinatorial applications to drug development versus fuel cell electrocatalysis. In-situ fuel cell spectroscopic studies, discovery level library preparation and screening, and focus level array fuel cell systems capable of high throughput fundamental studies will be discussed. Finally a perspective on near term future applications of combinatorial methods to fuel cell catalysts will be presented.
Manos Mavrikakis
Can Electronic Structure Calculations Aid the Rational Design of Catalysts?
Periodic self-consistent Density Functional Theory (DFT-GGA) calculations [1] have emerged as a valuable partner to experiment in explaining reactivity of transition metal surfaces. These calculations provide detailed atomic level mechanistic information on individual elementary reaction steps, in terms of reaction thermochemistry, paths, and activation energy barriers. Although the absolute numbers for heats of adsorption and activation energy barriers can be improved further, trends in reactivity derived from systematic investigations of specific steps on a number of different metal surfaces appear to be reliable, when compared to experiment. By focusing on a simple elementary step, such as O2 dissociation on transition metals, we show that there is a reasonably good correlation between the transition state energy and the final state energy of this step [2-6], allowing for the use of simple thermochemistry calculations to predict trends in the corresponding activation energy barrier. Combining segregation and other properties of multicomponent catalysts [1], with the above information, one can make progress towards identifying promising alloys in terms of facile O2 dissociation, without poisoning the surface with O atoms. Well controlled surface science experiments, combined with catalyst synthesis and classic catalysis characterization techniques could decrease the number of potential candidates for improved O2 activation catalysts further. In conclusion, we hope to show that reasonably expensive theoretical tools could aid in designing improved catalytic surfaces, with the additional benefit of an enhanced understanding of the fundamental reasons behind the success of these new catalysts.
[1] J. Greeley, J.K. Norskov, M. Mavrikakis, Ann. Rev. Phys. Chem. 53 (2002) 319.
[2] Y. Xu, M. Mavrikakis, Surf. Sci. 494 (2001) 131.
[3] Y. Xu, M. Mavrikakis, J. Chem. Phys. 116 (2002) 10846.
[4] Y. Xu, M. Mavrikakis, Surf. Sci. 538 (2003) 219.
[5] Y. Xu, M. Mavrikakis, J. Phys. Chem. B 107 (2003) 9298.
James A. Dumesic
Hydrogen from Catalytic Reforming of Biomass-derived Hydrocarbons in Liquid Water
The production of hydrogen from renewable biomass resources has environmental benefits as global energy production moves toward a "hydrogen society," because the CO2 produced during this reforming process is recycled during further production of biomass. Results from density functional theory calculations for various hydrocarbon fragments on metal surfaces suggest that the activation barriers for cleavage of C-C bonds are lower for adsorbed oxygenated hydrocarbons than for adsorbed hydrocarbon species. In addition, the thermodynamic properties of oxygenated hydrocarbons having a C:O stoichiometry of 1:1 are favorable for reforming at lower temperatures compared to reforming of alkanes. Accordingly, it should be possible to conduct catalytic reforming of oxygenated hydrocarbons at lower temperatures than currently required for reforming of alkanes. We demonstrate that H2 can be produced by reforming reactions of oxygenated hydrocarbons derived from carbohydrates with liquid water at low temperatures (e.g., 500 K). Aqueous-phase reforming over Pt-based catalysts leads to high selectivities for production of hydrogen (compared to the formation of alkanes), whereas reforming over Ni-based catalysts leads primarily to production of methane instead of hydrogen. Addition of tin to Raney-Ni-based catalysts suppresses the rate of methanation reactions, without significantly decreasing the rate of hydrogen production, such that the performance of Raney-NiSn catalysts compares favorably with that of platinum-based catalysts for production of hydrogen. We also show that the aqueous-phase reforming can be conducted at conditions that minimize the amount of CO (to levels of 60 ppm) in the product gas stream.
